
Chapter IV. The structure of the atom and the atomic nucleus. Using the energy of atomic nuclei
1. Which statement(s) are true?
A: isotopes have different masses of atomic nuclei
B: isotopes have different nuclear charges
2. Which statement(s) are true?
B: isotopes of the same chemical element contain the same number of neutrons
3. Which statement(s) are true?
A: isotopes of the same chemical element contain the same number of protons
B: isotopes of one chemical element contain different quantities neutrons
4. Among the proposed pairs of chemical elements, select those that are isotopes.

5. What is not the same for isotopes of one chemical element?
1) Number of electrons
2) Chemical properties
3) Nuclear masses
4) Charge ader
6. The number of electrons in an atom is
1) the number of neutrons in the nucleus
2) the number of protons in the nucleus
3) the total number of protons and neutrons
4) the difference between the number of protons and neutrons
7. The number of protons in the nucleus of an atom is
1) number of electrons
2) number of neutrons
3) the total number of neutrons and electrons
4) the difference between the number of neutrons and electrons
8. Total charge of electrons in a neutral atom
1) negative and equal in magnitude to the charge of the nucleus
2) positive and equal in magnitude to the charge of the nucleus
3) can be positive or negative, but equal in magnitude to the charge of the nucleus
4) negative and always greater in modulus of nuclear charge
9. Total charge of protons in the nucleus of a neutral atom
2) positive and modulo the total charge of the electrons
4) positive and always greater in magnitude than the total charge of electrons
10. Total charge of neutrons in the nucleus of a neutral atom
1) negative and equal in magnitude to the total charge of electrons
2) positive and equal in magnitude to the total charge of electrons
3) can be positive or negative, but equal in magnitude to the total charge of the electrons
4) equal to zero
on - negative. Therefore, the nucleus holds the electrons in the atom; The force of attraction towards the center causes the electrons to move around it.
These same electrical forces determine the sizes of atoms. When two atoms come very close together, enormous repulsive forces arise between their electrons. These forces prevent further approach and determine the volume occupied by the atom; no other atom can penetrate inside this volume.
Repulsive forces between atoms arise when the orbits (paths) of their electrons intersect. Therefore, the size of an atom is approximately determined by the diameter of its largest electron orbit (see Fig. 2).
ELECTRIZATION OF BODIES BY FRICTION
Why don’t we observe the electrical forces of attraction and repulsion between the bodies around us? After all, all bodies are made of atoms, and atoms are made of particles with electrical charges.
The reason is that atoms as a whole are neutral. The total negative charge of all electrons in an atom is equal to the positive charge of the nucleus. The total charge of an atom is zero. And since the atom is neutral, the molecule is neutral. And a body consisting of atoms or molecules is also neutral; it has no electrical charge.
Take a glass rod and rub it vigorously with a piece of dry silk. In this case, part of the electrons is separated from the glass molecules and goes to the silk molenules. The so-called ionization of some glass molecules occurs, their transformation from neutral particles into electrically charged particles - ions. Glass molecules that have lost one or more electrons are no longer neutral. The positive charge of the nuclei in such a molecule is greater than the negative charge of the electrons remaining in it. The molecule is positively charged and is a positive ion. An atom or molecule that has captured one or more extra electrons is called a negative ion.
If you touch two sheets of tissue paper suspended on threads with this stick, then some of the electrons from the sheets will be positively attracted
charged wand and switches to it. The leaves will become positively charged and begin to repel each other, as shown in Figure 3.
The leaves can also be charged negatively. To do this, instead of glass, you need to take an ebonite or wax stick, and instead of silk, use fur or woolen fabric. When rubbing sealing wax or ebonite with fur, some electrons transfer from the fur to the stick and it becomes negatively charged. The eletrons move away from each other. Therefore, when the stick touches the tissue paper,
Rice. 3. Yes, the charged pieces of paper are pushed away.
Rice. 4. They are differently charged
papers are attracted.
some of the eletrons are transferred to it. The two faces, which we touch with an ebonite or wax stick, are dressed up negatively. They are drawn away from each other in the same way as shown in Figure 3, and are attracted to the positively charged leaves (Figure 1). 4).
Nuclear energy: atomic energy is the internal energy of nuclear atoms released during nuclear reactions. Nuclear energy is based on the use of chain reactions of nuclear fission and thermonuclear fusion reactions.
Nuclear power plant (NPP) is a branch of energy that uses nuclear or nuclear energy. In the Soviet Union in 1943, the Atomic Energy Laboratory named after. V.I. Kurchatov, in which a nuclear reactor was built in 1946. The laboratory was renamed the Institute of Atomic Energy in 1955.
Nuclear radiation - originally particles and gamma rays emitted during the radioactive decay of nuclei. In the further flow of particles and gamma radiation from accelerators, charged particles, nuclear reactors, etc., as well as cosmic radiation.
Nuclear fuel is used to generate energy in a nuclear reactor. Usually it is a mixture of substances containing both fissile nuclei and nuclei capable of forming fissionable nuclei as a result of neutron bombardment.
The atomic theory of the structure of matter originated in ancient Greece. Much credit for the formulation of the scientific atomic hypothesis belongs to V.M. Lomonosov. He wrote that an atom is characterized by a certain mass, has chemical properties, and in molecules atoms are combined in certain quantitative relationships. In 1913, the Danish physicist Bohr, using the nuclear model of the atom as a basis, gave a detailed picture of the structure of the electron shell of the atom. He proceeded from the fact that the absorption and emission of light in an atom occurs in separate portions, quanta. From Bohr's provisions it follows that the farther an electron is from the nucleus, the greater the amount of energy it has. The atom, despite its insignificant dimensions 10"13 - 10""2 cm, is complex education. An atom is presented in the form of a nucleus consisting of heavy elementary particles - nucleons (protons - having a positive charge, and neutrons - having no charge), around which elementary particles - electrons - carrying a negative charge rotate at high speed. Protons and neutrons in the nucleus are tightly bound together through nuclear cohesion forces. In a neutral atom, the total charge of electrons is equal in magnitude to the total charge of protons. Electrons have a negative charge and therefore hold positively charged nuclei close to them. The mass of an electron is negligible and is 1/1240 of the mass of a nucleon. Gaining or losing an electron by an atom changes it Chemical properties, it is unstable and easily enters chemical bond with other atoms and molecules and is called an ion. The mass number of an atom is determined by the number of protons and neutrons in the nucleus. The number of protons for chemical elements is strictly defined and in the periodic table it indicates the atomic number. In the nuclei of atoms of the same substance, the number of neutrons can be different and they are called isotopes. In the periodic table they are in the same cell.
In 1898, M. Skladovskaya-Curie established that radiation is emitted not only by uranium salts, but also by the element thorium and its compounds. She and her husband Pierre Curie isolated two new radioactive elements from uranium ore, which were named polonium and radium.
Natural radioactivity is the spontaneous decay of a radioactive substance with the formation of a-b and y-radiation and a new substance with the release of energy.
The activity of a radioactive substance is a measure of the amount of radioactive substance expressed by the number of decays of atomic nuclei per unit time. The unit of radioactivity is the decay of an atom per second.
Curie is a unit of measurement of activity, symbolic designation C. I curie = 3.7 x 1010 decay events per second. The units of activity derived from the curie are 1 millicurn/1 mCurie = 0.001 curie microcurie /I mccurie 0.00001 curie/.
Becquerel - one decay in one second.
RADIUM - translated into Russian means RADIANT. Natural radioactive substances are elements that have the property of spontaneously emitting invisible rays. Radium emits three types of radiation, which were named after the first three letters of the Greek alphabet: a-rays, 0-rays, y-rays.
Alpha radiation is a stream of particles with a mass equal to 4 and a double positive charge. An alpha particle consists of two protons and two neutrons and is the nuclei of the element helium. Alpha particles arise during the decay of radioactive substances (natural radioactivity) or during the phenomenon of artificial radioactivity - in nuclear reactor. They have a very small penetrating ability, amounting to 50 - 70 microns in human tissue. but at the same time causing a high ionization density of 3-4 thousand ion pairs per unit path. In the air, one alpha particle forms 200 thousand pairs of ions. High ionization density results in high biological efficiency. Alpha particles carrying high energy (up to 800 MeV), obtained in nuclear reactors, have high penetrating ability.
Beta radiation is positively or negatively charged particles. They are formed during the decay of radioactive substances (natural radioactivity) or the phenomenon of artificial radioactivity in a nuclear reactor, as well as in linear or cyclic accelerators (linear accelerator, betatron). The penetrating ability of beta radiation generated during the decay of a radioactive substance in tissue is 8-10 mm. The ionization density of beta particles is 100 times less than that of alpha particles. At the same time, the flow of electrons can have great penetrating power, which is formed in accelerators and depends on the energy they have.
Gamma rays are electromagnetic waves whose properties resemble X-rays. The energy of y-rays, as a rule, is higher than x-rays, so the penetrating power is much greater.
Gamma radiation - electromagnetic oscillation, which occurs when the energy state of the atomic nucleus changes.
Table 4
Properties of radiation
|
Type, nature of radiation |
Speed |
Energy (E) |
Run length air - fabric |
Ionization density in tissues |
|
|
Helium nuclei |
3000-4000 ion pairs per 1 m |
||||
|
Electron flow |
87-298 thousand km/sec |
50-70 ion pairs per 1 m |
|||
|
Electromagnetic vibrations |
300 thousand km/sec. |
3000 ion pairs along the entire path |
|||
Atom is considered neutral, since its nucleus consists of particles: protons and neutrons. Each proton, although much heavier than an electron (1836 times), it also carries a unit charge. Just not negative, but positive. The neutron, as can be easily understood from the name itself, does not carry any charge at all: neither positive nor negative. The simplest example is the hydrogen atom, the first element of the Periodic Table. The nucleus of an atom of its isotope, protium (the most common), consists of a single proton. Accordingly, a single electron rotates around it in a circular orbit. Their charges cancel each other out, and the protium atom is neutral. Hydrogen also has other isotopes: deuterium (the nucleus of which, in addition to the proton, contains one neutron) and tritium (its nucleus contains a proton and two neutrons). These isotopes have slightly different properties from protium, but are also neutral. Each element of the Periodic Table has its own serial number. It matches the number of protons in its nucleus. Thus, silicon (Si) has 14 protons, manganese (Mn) has 25 protons, and gold (Au) has 79 protons. Accordingly, the nucleus of each atom of these elements “attracts” 14, 25 and 79 electrons to itself, causing it to rotate in circular and elliptical orbits. And atoms are neutral because negative charges are balanced by positive charges. Do atoms always remain neutral? No, very often they, having entered into a chemical bond with other atoms, either attract someone else’s electron to themselves, or give up their own. This depends on the so-called degree of electronegativity. If an atom attracts an extra electron, it becomes a negatively charged ion. If it gives up its electron, it also becomes an ion, but already positively charged.
An atom is like a miniature copy solar system. Only instead of the Sun, there is a massive core at its center, and instead of planets, elementary particles - electrons - rotate. An atom is electrically neutral, so the net negative charge of the electrons must be balanced by a net positive charge of the same magnitude. This happens because the nucleus consists of other elementary particles - protons and neutrons. Each proton carries the same charge as an electron, only with the opposite sign.
Instructions
You can find the number of protons in the nucleus using the periodic table. In this table, each element is assigned a specific, strictly defined place depending on its chemical properties. And chemical properties are, first of all, determined by the structure of the element’s atom.
Each cell of the table provides the necessary information about chemical element, including its serial number. This exactly corresponds to the number of protons in the nucleus of an element’s atom.
Look at the table. Element number 11 is the alkali metal sodium (Na). Therefore, there are 11 protons in each sodium atom. Or element number 23 - the metal vanadium (V), which exhibits not only basic but also acidic properties in its compounds. Based on their serial number, we can conclude: each vanadium atom contains 23
The nucleus of an atom contains protons that are positively charged (+).
Around the core A negatively charged electrons rotate(-).
The moduli (values) of the charges of protons and electrons are equal.
The number of electrons in a neutral atom is the same as the number of protons in the nucleus. Therefore, the total charge of such an atom is zero.It is clear that the charge of a body consisting of such neutral atoms is also equal to zero - it also has the number of “minuses” - electrons - equal to the number of “pluses” - protons.
If a body has the number of charges of one sign and the number of charges of the opposite sign, it is charged.
Note that p rotons are bound by atomic nuclei and cannot leave the body, and electrons located in the orbit farthest from the nucleus (valence electrons) are quite capable of escaping from the atom. Therefore, the charge of a body depends on how many electrons have left it. Or how many excess electrons a body contains.
If some electrons leave the body, it turns out that more protons remain in it. Therefore, the body is positively charged.If there is an excess of electrons on a body, the body is negatively charged.
The greater the excess or deficiency of electrons on a body, the greater its charge.
Charge of both proton and electron elementary(minimum existing in nature), equal to 1.6*10^-19 C. Nothing can have less charge.
That's why the charge of any body can only be a multiple
1.6*10^-19 Cl, i.e., it does not change smoothly, but discretely (abruptly), depending on exactly how many extra electrons a given body contains, or how many are missing. One electron is missing - the charge of the body is1.6*10^-19 C, one extra electron - the charge is minus1.6*10^-19 C (electrons are negatively charged). Three electrons missing - charge 4.8*10^-19 C and so on.A
1 coulomb is a total charge of 6.24*10^18 electrons (or protons).So they decided: the charge of exactly this number of electrons is called 1 coulomb.
A liter of water contains approximately 3*10^25 molecules. We don’t say: pour me a drink 3*10^25 water molecules, we say: a liter, half a liter, one and a half liters, etc. Same with the pendant. Pendant - a measure of measurement large quantity elementary charges, just like a liter (or mole) is a measure of a large number of molecules.
By the way, the mass of water also changes discretely: one molecule is added - the mass of water changes to the mass of this very molecule - abruptly. Both water and charge cannot be divided into parts endlessly, because there is a minimal unit of both.
It is not difficult to figure out that two numbers are the charge of one electron
1.6*10^-19 Cl and 6.24*10^18 - the number of electrons whose total charge is 1 Cinverses: multiplying them we get one.
Analogy: one company - 100 soldiers. One soldier is one hundredth of a company. These numbers are also reciprocals of each other. Multiplying one hundredth by one hundred we get one:
0.01 x 100 = 1.
Charged body creates around itself electric field, and reacts to foreign electric fields as follows:
attracts objects with opposite charge(a body with an excess of electrons is attracted to a body with a deficiency of electrons) And repels from bodies charged in the same way as itself(minus is repelled from minus, plus from plus).In general, he behaves heterosexually.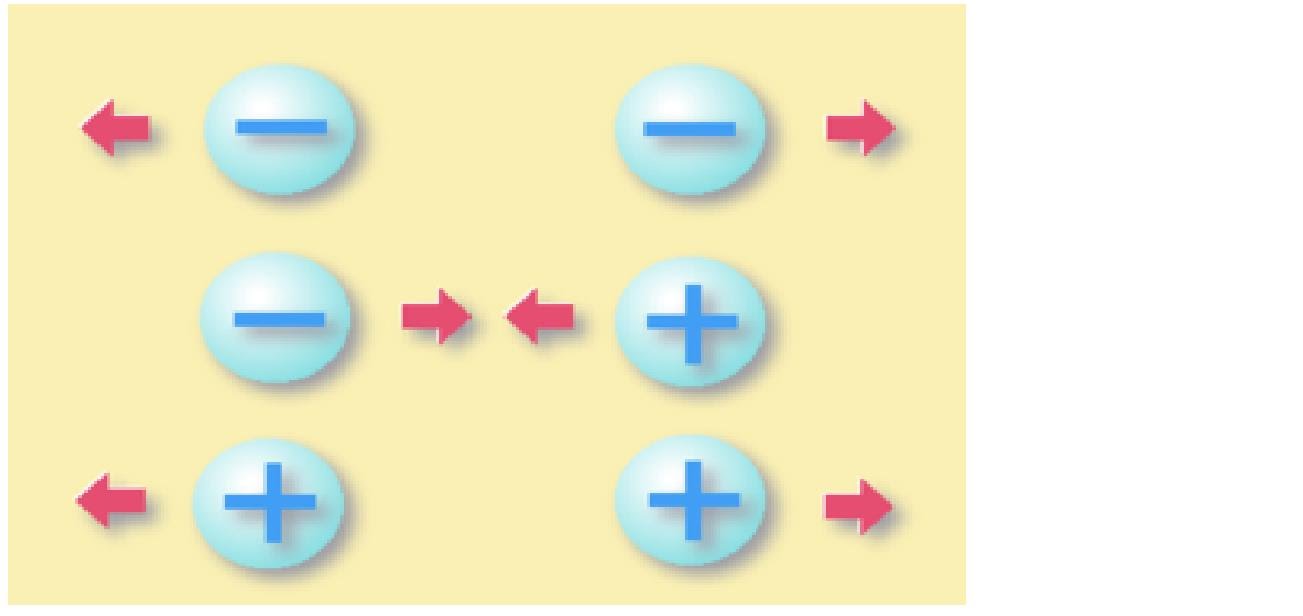
A charged body also interacts with a magnetic field, but only when the body moves relative to magnetic field. The greater the charge of a body, the stronger it interacts with electric and magnetic fields.
The easiest way to electrify a body is by rubbing it against another.
As mentioned above, charged bodies either attract each other or repel each other.
The strength of their interaction is described by Coulomb's law
The force of interaction between two bodies F is directly proportional to the product of their charges q 1 q 2 and is inversely proportional to the square of the distance r between them. The charge of one body (in coulombs) is multiplied by the charge of the second body. And divide by the square of the distance between them (in meters). We multiply the result by k. In different environments, the strength of interaction between charges may vary." k"in Coulomb's law - a coefficient for specific environments in which charges are located. In a vacuum there will be one k, in water - another.
Let's repeat: 1 coulomb is a charge of 6.25 * 10^18 electrons. If this is how many electrons a body lacks, its charge is 1 coulomb (1 cell). If there is the same excess of electrons, the charge of the body is minus 1 cell (electrons are negatively charged). It is clear that if the excess of electrons, for example, is 10 times less, the charge of the body is, accordingly, minus 0.1 cells. Not difficult.
The formula shows that if at least one body is not charged (q1 or q2 = 0), there will be no interaction force.
Why does the force of interaction decrease in proportion to the square of the distance r between them? Because the area of the sphere is proportional to the square of the radius:
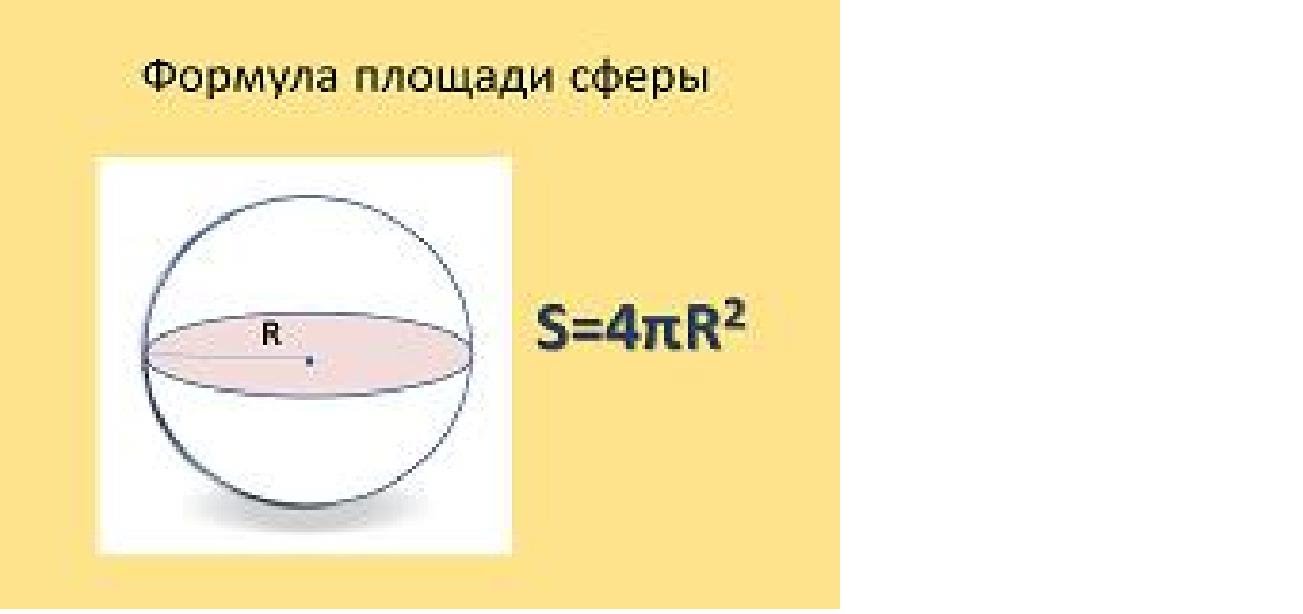
According to the same law, the shock wave of an explosion decreases: if a meter from the place of the explosion the wavedistributed over an area of 4 * 3.14 * 1^2 = approximately 12 sq.m., then in two meters, the explosion energy is spread over 48 sq.m.: for each unit of area it will be received four times less - when the distance is doubled . The picture is the same with the electric field of a point charge. When the distance between charges doubles, the interaction force decreases fourfold. When the distance increases ten times, it decreases by a hundred.
Why are the charges in the formula multiplied and not, say, added?
If we, for example, double the charge of one of the interacting bodies, this will mean that the number of missing or extra electrons on it has doubled. And the force from the second body acts equally on each elementary charge. This means that the force of interaction will double. Approximately how the Earth attracts a two-kilogram weight twice as strongly as a kilogram weight. Which, in fact, is reflected by Coulomb’s law - force is a multiple of charge.
Note that Coulomb's law practically copies the law of universal gravitation:
There is also a coefficient, the product of two masses (instead of the product of two charges) and the square of the distance between them.
An electric field is created around charged bodies. An electric field is something that affects charged bodies whether they are moving or not, unlike a magnetic field, which acts exclusively on moving charges. But more on that later.
By the way, a person is able to determine the presence of a strong electric field. If you pass the back of your hand near a highly electrified body, you can feel the hairs moving on it. If they are.
Like any physical phenomenon, the electric field needs to be measured somehow.If you try to lift a weight on Earth, for example, 16 kg, you will notice that the Earth is attracting it with some force. The Moon attracts the same weight with a force approximately 6 times less.And in zero gravity, the weight (but not the mass - a measure of inertia!) of the weight completely disappears.By measuring the force with which various planets attract a weight, their gravitational force can be determined.
The same approach is used to measure the parameters of the electric field: it is judged by the force with which it attracts (or repels) the test positive charge:
E = F/q.
Electric field strength E proportional to force F, acting on a point charge q. (Point charge - concentrated on a small body, the dimensions of which can be neglected).The stronger the field pulls or pushes the same charge, the greater the intensity of that field.We measure force F in newtons, charge q in coulombs. Therefore, the unit of measurement of electric field strength E isnewton/coulomb.
On the left is a body with a mass of 102 grams in the Earth's gravitational field. On the right is a weightless body carrying a charge of 1 coulomb, located in an electric field of intensity 1 newton/coulomb. Both bodies are acted upon by forces of the same magnitude - 1 newton. The left body attracts the Earth's gravitational field, the right - the electric field.
Let me remind you: 1 newton is the force capable of accelerating a body weighing 1 kg by 1 meter per second for one second. 1 newton = 1kg*m/s^2.If a body weighing 1 kg accelerates by 1 m/s, in 1 second, then the force acting on the body is equal to 1 newton. A ten-kilogram body accelerated by 3 m/s per second - a force of 30 newton (10 kg*3 m/s^2 = 30 N).
Since the charge on the spring is positive and pulls it down, it means that the lines of force of the external electric field are directed from top to bottom. That is, plus fields at the top, minus at the bottom. The probe is repelled by the same charge and drawn to the opposite one.
Why does the field strength formula look exactly like this? What if we take a test charge, say, twice as much?
Then the force with which the field acts on the charge will increase exactly twice. This means that the ratio of the force acting on the charge and the magnitude of this charge will remain the same:E = 2 F/2q= F/q.
We can write this formula differently:F = E*q.
The force acting on a charge at a given point in the field is proportional to the field strength and charge - just like the weight of a body(force of pressure on the support)on a given planet depends both on the gravity of the given planet and on the mass of the body.
Another blow to the lid: q = F/E. In this form mIt is possible to calculate the magnitude of the charge by the force with which an electric field of known strength acts on it (as you can calculate the mass of a body by measuring the force with which it is attracted by the Earth - in fact, the scales show this force). We divide the force in newtons by the tension newton/coulomb. Reducing newtons, we get the answer in coulombs.
As in the case of measuring gravity, the test charge must be small so as not to introduce distortions into the electric field itself.
Indeed: a kilogram weight will allow you to measure the gravity of the earth. But if you try to use the Moon as a weight, the influence of its mass will become noticeable. But we need to find out with what force the Earth attracts objects (measure its gravitational field), and not with what force two massive bodies attract each other.
If you bring it to a charged body test charge - a small body with a positive charge(hereinafter referred to as “probe”), the latter will either be attracted to the body (if their charges are opposite) or repelled from it. Moreover, The probe will move towards or away from the body along a certain trajectory.The trajectory of the probe is called the electric field line.
Field lines are drawn with arrows indicating the direction in which the probe will move. Bodies of different shapes have different lines of force:single point charges - radially diverging or converging straight lines (a).The field lines near the sharp protrusions of charged bodies have the same shape.
If there are bodies nearby with charges of opposite signs, part of the lines of force begins on positive charges and ends on negative ones (b).
For point charges of the same name, the lines are approximately the same, but “diverge” in the zone between the charges (c).
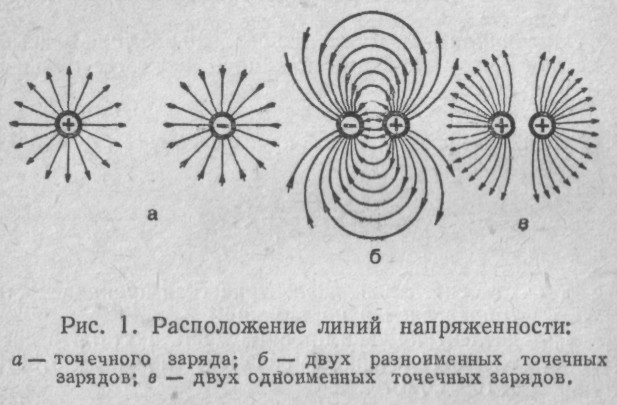
Don't forget: lines of force show the trajectory of a positive test charge.
It is noticeable that the line density decreases with distance from the charges. But this doesn't always happen.
Remember the example with the explosion? The power of the shock wave decreases in proportion to the square of the distance. But if the explosion occurs in narrow corridor or a mine, a shock wave can travel quite far without weakening - simply because it has nowhere to dissipate: the cross-sectional area of the corridor (and therefore the shock wave) does not change with distance.
The same picture occurs near a flat plate of large area. The lines of force are parallel to each other, and the electric field (its strength) E doesn't change quite long distance from her.
Superposition principle
The thing is obvious. If we had another Earth under our feet, the force of gravity would double. If it’s two, it’s tripled. AND It is a known fact: the gravitational force of the Moon “breaks through” to the Earth, causing ebbs and flows in the coastal zones of the oceans. This phenomenon is not hampered by the presence of its own gravitational field Earth. PThe superposition principle states: the electric field of each body extends in space regardless of the presence of other electric fields around it . The fields do not influence each other, but are simply summed (added).Let's return to the drawing with two charges of the same name:
It seems that it is clear that the fields of these two bodies influence each other? Otherwise, why are the field lines so crooked?
Let's try to understand why the lines have this shape. Let's say the probe (small gray circle in the figure) is located on the surface of the right body, at the point where its upper left field line begins.Since the body and the probe are charged equally, the latter will be repelled from the surface of the body and will begin to move in the direction from the center of the right body, perpendicular to its surface. At this moment, the influence of the right body on the probe is great (since it is close), the influence of the left body is not yet noticeable (we remember: the fields of point charges decrease in proportion to the square of the distance from them). As the probe moves away from the right body, the influence of the latter decreases, but the influence of the left body (which also repels the probe) increases. As a result, the probe has “nowhere to go” except up: it is pushed from both the right and the left. This is what the force lines demonstrate. The individual lines of force of both bodies were and remain radial (directed from the center to the sides, like the rays of the Sun).
You can depict the forces acting on the probes in the form of vectors (arrows). The direction of the vector will show the direction of the force, the length of the vector shows the size of that force.
Forces acting on probes from the body A designated a, acting from the body B, respectively, b. Vector c- resultant (sum of vectors a And b). Let's remember how vectors add up and add up the forces acting on the probes.
On a straight line between the centers of bodies A And B the vectors cancel each other out. Their sum is zero. And the test positive charge located at the midpoint will not move either to the left or to the right. It is understandable even without addition - it is pulled to the left and to the right equally. (If this probe was initially not in the center, it will still be pushed to the center, to the point of “equilibrium” because the near body pushes away stronger than the far one). If the probe is closer to the body A(second from top in the picture), vector a, depicting the force acting from the side of this body is long (since the force acting from the side of a close body is greater). Vector b force acting from the body B, short. Summation vector c shows the direction of the resulting force, and therefore the direction in which the probe will move. A the direction of movement of the probe is the power line.
Exactly the same picture will occur between unlike charges. If you want, add the vectors, if you want, follow the movement of the positive probe at each point - the result is the same.
The probe flies away from the left body perpendicular to its surface. As you move away from the left body, the influence of the latter decreases, but the influence of the right one, attracting the probe, increases. Therefore, the trajectory (path) of the probe bends in the direction of the right body. Hence the curved lines of force. You can notice that the lines of force are tangents to the arrow vectors.
If our charged bodies are placed in any dielectric, the field strength around them (or between them) will decrease several times:E = E 0 / ε, Whereε - coefficient of dielectric constant, showing how many times the tension has decreased compared to vacuum.
What does this give in practice?ε? Reducing the electric field strength means that our favorite probe will be subjected to less force than in a vacuum. How many times less - showsε. If this design
transfer from vacuum to distilled water (ε water = 81), the right spring will stretch 81 times less! Dielectric reduces charge interaction. If you place a dielectric with a largeε between the plates of a capacitor (a device that stores charge), this capacitor can store inε times more energy.
Surface charge density
Let's remember what bodies look like at high magnification:
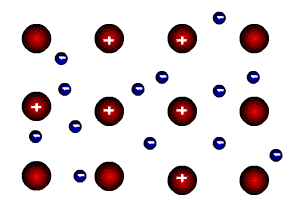
Red balls are atoms that are positively charged (because some electrons have left them), blue balls are negative electrons.If the number of protons in a body is equal to the number of electrons, the body is not charged. If a body has, for example, an excess of electrons, it is negatively charged. But there is an interesting point -all excess electrons will be forced out to the surface of the body!There is no charge inside the bodies.
This happens for a simple reason: extra electrons repel each other because they are similarly charged. Where will the electrons be? maximum distance from each other? On the surface of the body, where else?
If there are not enough electrons in the body (the body is positively charged - there are more protons than electrons), the picture is the opposite - I The nuclei will attract electrons, driving them inside. Outside t e There will be a shortage of electrons. But this means that the surface of the body is positively charged. Charge on the surface again!
The above applies to conductors (substances in which electrons are not tightly attached to atoms) - in them electrons have the ability to move in or out. Nevertheless, with insulators(insulators have stationary electrons) the same picture - the charge is always outside. And precisely because of the impossibility of moving electrons: electrons are either taken from the surface of the insulator, or extra electrons are introduced onto the surface of the insulator.And if so, physicists introduce the concept of “surface charge density”.
σ =
The surface charge density of a body is equal to the ratio of the charge to the surface area of the body.
We remember: charge is what electrons and protons carry. It is clear that two bodies with the same charge(i.e. the same excess or deficiency of electrons) may have different surface charge densities - if they have different area surfaces. Electrons can be “smeared” over a large area, or concentrated on a small spot.
Looking ahead, we note that it is the shape of the body that determines its electrical capacitance- how “cramped” are the electrons on it. And the more electrons collected per unit area, the higher it will be potential bodies.
Two differently charged plates
are called electric capacitor. Their electric field looks like this:
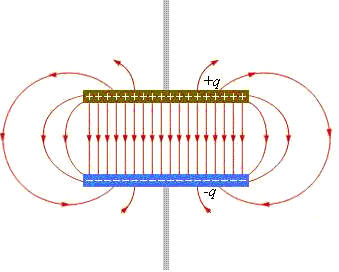
Between the plates, the power lines go, of course, from plus to minus, but above and below the plates there is no electric field, because the fields of one plate compensate for the fields of the second plate.
An electric capacitor stores not the electrons themselves, not their excess or deficiency, but the difference: how many electrons are missing on the positive plate (called the plate), the same number of extra electrons on the negative plate. To charge a capacitor, you need to transfer some electrons from one plate to another.
Electric dipole
These are two identical point charge, spaced apart and rigidly connected to each other. A design, like a dumbbell, in which one ball is positively charged, the second is negatively charged.
Since the charges are separated, the dipoles react to the external electric field (they turn with the positive end along the field) and interact with each other - they attract the oppositely charged ends of their neighbors.
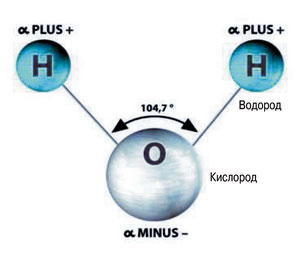
Similar designs with spaced charges exist in nature. Water molecules are just such dipoles (“di” means “two”) - two connected spaced charges ( in a water molecule there are three separated charges, but two plus charges can be represented as one double charge, located approximately in the middle between them).
In a water molecule H 2 The O electrons connecting the atoms are shifted towards the O oxygen atom. Therefore, the oxygen atom is negatively charged. The hydrogen atoms H are deprived of electrons and therefore are positively charged (only protons remain from the atoms).
Due to dipole, water molecules briefly stick together into clusters - groups of molecules - the positive ends of some molecules are attracted to the negative ends of other molecules:
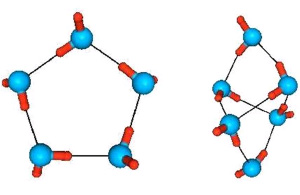
The dipole nature of water molecules explains the high coefficient of its surface tension. Water molecules behave like a bunch of magnets - they stick together. And if the surface next to the water also has such “magnets” - polar molecules, then the water will wet such a surface. If not, water molecules. You can also use this effect.
Conductors
Atoms of some substances weakly retain their electrons, which are located in the most distant orbits(valence electrons). Such substances are called conductors. Electrons separated from atoms are able to move inside a conductor. And since there are charged particles capable of moving, these substances conduct current, sincecurrent is the ordered movement of charged particles. Actually, the name “conductor” means the ability to conduct electricity. In particular, metals are conductors:
The general condition for a substance to belong to conductors is the presence in them of free (capable of moving within the substance) charged particles. In addition to electrons, such charged particles can be, for example, ions in ionized gases and aqueous solutions of salts and acids.
Molecules table salt NaCl, when dissolved in water, splits into ions: Na+ and Cl-. A sodium atom, having given one electron to a chlorine atom, turns into a positive ion, and chlorine, with an extra electron taken from sodium, turns into a negative ion. And if you put two wires into an aqueous salt solution and apply voltage to them, the sodium atoms Na+ move in the direction of the “minus” (negative electrode), chlorine atoms Cl- in a positive direction. It’s clear why: opposite charges attract. The sodium atom is missing one electron; it can get it at the negative electrode. The chlorine atom can shed an extra electron at the positive electrode. Upon reaching the electrodes, both types of ions are converted into the starting substances - sodium and chlorine. But we digress, because now we are not talking about electrolysis, but about electric current - the movement of charged particles. The movement of chlorine and sodium ions in the direction of the electrodes is an electric current.
Conductors in an electric field.
We remember that a conductor contains mobile charged particles. We also know that opposite charges attract, and like charges repel. Based on this, you can guess what will happen in the conductor when it is in an electric field.
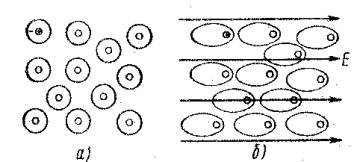
The left picture shows the metal in the absence of an electric field. Positively charged nuclei and free electrons are distributed evenly. It cannot be otherwise: if there is an excess of electrons in some area of the metal (such a short-term local change in the electron concentration is called a fluctuation), they, due to mutual repulsion, will quickly leave this place. If there is a local shortage of electrons, this will mean that there are more positively charged nuclei there. And electrons will be attracted to this region by Coulomb forces.
When an external electric field appears (middle picture), the electrons, of course, will move in the direction of the “plus” of this field, that is, to the left (field lines, let me remind you, are drawn from plus to minus of the field). But. Since some of the electrons “went to the left,” an excess of positively charged nuclei appeared on the right. That is, an electric field of its own was formed inside the metal due to the movement of some electrons. And since the “plus” of this own field is on the right, and the “minus” is on the left (where the electrons are collected), it means that the conductor’s own electric field is directed counter to the external one. And at the moment when the internal field becomes equal to the external one, the movement of electrons will stop (the right picture shows the equality of the external and internal fields). It is clear that the stronger the external electric field, the more electrons will shift to the left.
The redistribution of charge carriers under the influence of an external electric field is called electrical induction.
It is clear that by turning off the external field, we will restore the status quo: electrons will leave the left surface and be evenly distributed throughout the conductor.
Note: if a conductor is divided in an electric field in two (across the field), each half of the conductor will be charged: the half on the plus side of the field will have an excess of electrons, and the other half will have a deficit.
If there is a cavity (emptiness) inside the conductor, there will be no electric field in it - precisely because of the compensation of the external electric field by the conductor’s own field. The internal cavity in the conductor is protected (they say “shielded”) from external fields. Electrostatic protection is based on this: objects are placed in a grounded (connected by a conductor to the ground) metal shell, not necessarily solid; a mesh (the so-called “Faraday cage”) is also suitable. One of the “mythbusters” Adam Savage demonstrates the effect of such protection very effectively.(Adam Savage):
The voltage of artificial lightning can be judged by the following fact: the dielectric strength of air is 3,000 volts per millimeter - if a voltage of 3,000 volts is applied to electrodes located at a distance of one millimeter from each other, an electrical breakdown will occur between them - electric arc. Accordingly, to break through one meter, it takes a thousand times more - 3,000,000 (three million) volts. Let us remind you that 220 volts in a household electrical network is enough to kill a person. However, Adam seems to do well with lightning strikes that strike the cage clearly longer than two meters.
By the way, the music in this video is played by the lightning itself: the voltage to the Tesla coils is supplied from an audio amplifier. The air in the electrical breakdown channel expands due to heating and ionization, creating sound. Lightning strikes look even more impressive inconductive suit.
Dielectrics in an electric field.
In order for a substance to conduct current, that is, so that charges can move in an orderly manner, the presence of carriers of this same charge in the substance, moreover, mobile ones, is required. But dielectrics don’t have them. More precisely, there are charge carriers themselves ( any matter is made up of atoms, and atoms contain positively charged protons in nuclei and negatively charged electrons in orbit around the nuclei), but these carriers cannot move through the dielectric. In dielectrics, electrons are tightly held by atoms, and there are very few free electrons.You can read about the reasons on the page ".
When heated, the conductivity of dielectrics increases: temperature is a measure of the speed of movement of atoms and electrons of a substance. The faster you move the atoms and electrons of a substance are fused, the higher its temperature. That's why larger number electrons break away from atoms (how highly accelerated satellites can leave the earth’s orbit) and become free (which means they can transfer charge - conduct current).
When heated, metals, on the contrary, conduct current less well. In metals, even at low temperatures, there are enough free electrons to ensure conductivity. As the temperature increases, the amplitude of vibrations of atoms fixed in the nodes of the crystal lattice increases, and it is more difficult for electrons to break through this lattice.
Polar and non-polar dielectrics.
What does an atom of any substance, such as a hydrogen atom, look like? This is a proton in the nucleus and an electron rotating around the nucleus at such a speed that we can say that the “minus” of the atom is smeared around the “plus”. The "centers of gravity" of both charges coincide. The properties of such an atom are the same in all directions - it is a sphere.
If such an atom finds itself in an electric field, what will happen to it? Probably the nucleus of the atom will shift along the field (in the negative direction, like the test charge), and the electron cloud will move in the opposite direction?
Exactly. This is exactly what happens. Now our atom has poles: negative on the left and positive on the right. That is, the atom has become polarized. This type of polarization is called electronic or strain polarization. The meaning is clear: electronic - because the electron cloud has shifted relative to the nucleus. Deformation - look what the electric field did to our ideal ball: it deformed it, flattened it.
Now let's take a water molecule. It initially has poles, since the oxygen atom attracts electrons from both hydrogen atoms. Therefore, the oxygen atom becomes the negative pole of the molecule, and the hydrogen atoms (more precisely, the point approximately in the middle between the hydrogen atoms) become the positive pole.
And since a molecule has positive and negative poles, it is clear that in an electric field it will turn plus to minus, minus to plus of the field:
This type of polarization is called orientational (polarization due to the orientation of molecules).
It is clear that when the external electric field is removed, the position of the molecules will become disordered.
However, there is a trick: if you polarize such a dielectric in liquid form and then let it solidify, the molecules will not be able to return to a chaotic state. A dielectric that maintains polarization for a long time is called an electret. The electret itself creates an external electric field. You can read more.
Another type of polarization is ionic polarization. It is usually demonstrated using sodium chloride crystals: NaCl:
Salt crystals consist of positive sodium ions and negative chlorine ions (why this is so - on the page in the section "ionic bonding").
It is clear that in an electric field, sodium ions will move along the field, and chlorine ions will move against the field.
From the above, we can draw a seditious conclusion: dielectrics do conduct electric current. After all, what is current? Current is the ordered movement of charged particles. What happens during the process of polarization? This is exactly the kind of mass directed movement. The only difference is that the movement of charges in dielectrics is limited by the boundaries of the atom during deformation polarization and by the rotation of molecules during orientational polarization. Well, and the displacement of atoms in lattices during ion polarization. That is, current flows, but very short-term. Until the charges “hit the wall” - exactly the same as what happens during the polarization of conductors in an electric field (it’s called electrical induction, see above).
Such currents are called polarization- current only at the moment of polarization of the dielectric.If we very quickly and often change the direction of the external electric field, then, due to the constant change in the direction of polarization, a current will flow in the dielectric. Clearly, extremely variable. It is polarization currents that heat food in a microwave oven.
When dielectrics are polarized, negative charges appear on their surface (and only on the surface) facing the positive side of the external field, and positive charges appear on the surface facing the negative side of the external field.
These charges are bound (with the molecules of the substance), that is, they cannot be removed from the surface.
Inside the dielectric, the total charges are zero, and the electric fields of polarized molecules are directed against the external electric field. This shows another analogy with conductors. But if there is no electric field inside the conductor, it is present inside the dielectric, although weakened several times. For example, in distilled water (we remember, it has orientational polarization), the electric field decreases by 81 times. This the coefficient of attenuation of the external electric field is called the dielectric constant.
The dielectric constant
Let's take two differently charged plates. The field lines between them are directed from plus to minus, the length of the arrow lines symbolizes the magnitude of the field strength.
Now imagine that between the plates we have certain structures in the form of spaced apart point charges-balls on sticks (electric dipoles), capable of rotating around their own center of gravity.
If our plates are charged, these structures will unfold in a clear way: plus to the minus plate, minus to the positive. What happens now? The electric field created by the plates is superimposed by the electric field present between the balls on the stick (short arrows along the sticks). And this field is directed opposite (counter) to the field created by the plates. And if so, the field strength between the plates will drop! Therefore, in the right figure, the arrows between the plates are shown shorter.
If you pour distilled water (it consists of dipoles) between charged plates, the water molecules will turn with oxygen atoms towards the positive plate, and hydrogen atoms towards the negative plate. The interaction (attraction) of the plates will decrease, as will the electric field strength between them (the effect on the test charge). And 81 times! This number is 81 and is called dielectric constant of the dielectric. It shows how many times the interaction of charges in a given dielectric is weaker than in vacuum.
This figure is different for different dielectrics.- it depends on specific location atoms in polar dielectric molecules. Table of dielectric constant of some substances - .Work of electric field forces.
A charge in an electric field is acted upon by a force. If the charge, succumbing to the action of this force, begins to move, it means that the field is doing work. How else? If something happens, then someone (or something) is working.
In physics work is equal to the change in the energy of the body - the object of application of work.
For example: the kinetic and potential energy of a brick lying on the ground is zero. If we kick a brick then Let's give it kinetic energy - the energy of movement. It is equal to the product of the mass m of the brick (in kilograms) by the square of its speed v (in meters per second) divided by two E=m*v^2/2.The result obtained, measured in joules (J), is equal to the work we have done (and the acceleration of the brick, i.e., the transfer of energy to it, is the work) A, also measured in joules.
If we raise the brick to a certain height, the work done will be equal to the potential energy of the brick at this height: E=m*g*h. We multiply the mass of the brick in kilograms m by the gravitational constant g (rounded to 10) and by the lifting height in meters h. And in this case, the energy of the brick will be equal to the work done A in the same joules. It can be seen that the greater the mass of the brick, the force of gravity on a given planet and the height of the lift, the more work we do. our workdescribed by the formula
A = m*g*s*cos a
The work is equal to the product of the mass m, the acceleration of gravity (also known as the gravitational constant of the Earth) g, and the distance traveled s. More about cosine below.
Now let's look at the formula that describes the work of the electric field when moving a charge, at the part of the formula that is after the second equal sign:
Electric field work A is equal to the product of charge q, field strength E and distance change delta L.
Everything is the same as our brick: the work of the gravitational field A is equal to the product of mass m (its analogue in electrostatics is charge q), the gravitational constant g (analogue is field strength E) and the change in height delta h (distance L).
There is also a cosine in the formula. You can read about it on the page. Its meaning is this: we can lift our brick vertically (along the line C B on the left triangle), or drag it along the inclined plane A B to the same height. Regardless of the lifting trajectory, the height of the brick at end point B, and therefore its potential energy, will be the same.Consequently, the work we do is the same in both cases.
However, the path of a brick up a gentle hill is longer than the vertical climb. If we stupidly substitute the path traveled s into the formula
A = F*L*cos a
ignoring the cosine, it turns out that the flatter the hill (the longer our path), the more work we have done. But this is not true (a flat path is easier, although it is longer). Cosine just like that shows how many times a straight path is shorter than a shallow one lift (for a given angle). Let's say our slide is twice as long as the straight path. This ratio occurs when the angle between the vertical and the slide is 60 degrees (see the cosine table). The cosine of an angle of 60 degrees is equal to 1/2, or, which is the same, 0.5.Let's say the lift height is 3 meters. By lifting the brick vertically, we substitute these 3 meters (s) into the formula. The cosine in this case is equal to one (cos 0 = 1).
When lifting a brick along a six-meter slide, we multiply its length by the cosine of the angle between the plane of the slide and the vertical, that is, by 1/2, and in the end we get the same three.
Now everything fits: regardless of the trajectory, lifting the brick to the same height means doing the same job.
Using the cosine table, you can find out how many times leg C B is shorter than the hypotenuse A B for any angle, for example, for a right triangle with an angle of 45 degrees.
But fiddling with cosines only makes sense if there is a straight trajectory of movement of either a load or a charge. More often the trajectory is more complex. However, as mentioned above, the work depends only on the difference in potential energies of the manipulated object - brick or charge - at the initial and final points of the trajectory.
Since we're talking about bricks, let's imagine the situation: your job is to deliver bricks from the bottom floor of a building to a bricklayer working on the second floor. The mason does not care how the bricks get to him: on a vertical lift, along a fire escape or access ladder, or even in transit through the ninth floor. Only the result is important to him - the brick was originally lying on the ground, and now - here it is, in his hands. And you will receive a salary for your work regardless of pretzels and bricks: solely based on the result. That is, according to the difference in brick energies.
It is clear that if a brick moves downward, the work of moving it is done by the gravitational field. At the same time, the potential energy of the brick decreases.
The above applies fully to the movement of a charge in an electric field: the work of electrostatic forces when moving a charge q in an electric field is equal to the decrease in the potential energy of this charge:
A1-2 = Wp1 - Wp2 = q φ1 - q φ2 = q(φ1 -φ2).
In the same way, the measure of the work of the earth’s gravitational field is the decrease in the potential energy of the body: A 1-2 = W p1 – W p2.
A body of mass m (in kilograms) at a height g (in meters) has potential energy (in joules)equal to m*g*h, where h is the height of the body above ground level. Since at ground level the height h is zero, then the potential energy of the body at zero height is equal to the same zero(W p2 =0): multiply the product m*g by zero (it is logically understandable: a body at zero height cannot do any work. It has no energy). Therefore, work gravitational forces when moving a body from height h to zero it is equal to m*g*h - 0 = m*g*h = W p1. In short: A = W p1.
Likewise, the work of electrostatic forces when moving a charge q from a point where a given charge has potential energy to a point with zero potential energy is equal to
A = W p1 = q* φ .Another aspect: our brick lying on the ground does not have potential energy only in relation to the surface of the earth. But imagine. that a well was dug in the ground. Relative to the bottom of the well. the brick will already have energy, and falling there can do a lot of business.
But also brick. lying at the level of the second floor has energy only relative to the ground. relative to the second floor, its energy is zero. Conclusion: potential energy depends on the reference point. It depends on what level we take as zero.
Electric field potential.
The textbooks say: electric field potential - a scalar quantity determined by potential energy Wpunit charge q placed at this point φ = W p /q.
Let's return to gravitational-mass analogies.Let us determine the potential of the gravitational field. Let's call it the same:φ. WITH using the gravitational formula we will perform the same trick, not forgetting that the analogue of charge q for gravity is mass m
φ = Wp/m:
the potential of a point in a gravitational field is equal to the ratio of the potential energy of a body to the mass of this body. But sinceW p = m*g*h (formula for potential energy of a body), it turns out that the field potential φ = m*g*h/m.
But - to practice. The “point of the gravitational field” will be, for example, the window sill of a second floor window at a height of 5 meters from the ground. How to determine the potential of this point?
Let's take our favorite brick. Let its mass be 2 kg.
φ = W p /m =m*g*h/m.
We multiply the mass of the brick (2 kg) by the acceleration of gravity (roughly 10 m/s^2) and the height (5 m). We divide the result (100) by the mass of the same brick (2 kg). We get: the gravitational potential at the window sill level is 50.
And 50, actually, what? In what units do we measure? In which ones you can measure, these are the ones you can measure in:
kg*m/s^2*m/kg.
The kilograms in the numerator and denominator will be reduced. Meters too. The square of the speed will remain in the denominator: 1/s^2. The potential is 50/s^2.
"Wikipedia" agrees with us: “Gravitational potential is a scalar function of coordinates and time, characterizing the gravitational field in classical mechanics. Has the dimension of the square of the speed, usually denoted by the letter φ . Gravitational potential is equal to the ratio of potential energy material point, placed at the considered point of the gravitational field, to the mass of this point."
Note that we could do just fine without a brick: what’s the point of multiplying by its mass and then dividing by it? Let's reduce the mass in the numerator and denominator:
φ = m*g*h/m = g*h.
Potential equal to the product acceleration of free fall and height of a given point:
φ = g*h.
The main question is: why the hell? Why do we need to know the gravitational field potential?
It’s simple: the energy generated by a hydroelectric power station, for example, depends on this figure - the greater the height and acceleration of free fall at the point of water intake, the more energy the power plant will produce. And one should not think that the acceleration of free fall is a constant value: it depends on the location of rocks in the earth’s thickness, on geographical latitude(The Earth rotates, therefore, the acceleration of gravity at the equator is less) and even on the time of day and the position of the Moon - sea ebbs and flows are precisely caused by the fact that the potential of the gravitational field “walks” due to the superposition of the gravitational fields of the Sun and Moon on the field Earth (remember the principle of superposition).
However, it's time to return to electricity.
Remember, the electric field parameter “tension” is equal to the ratio of the force acting on a charge to the magnitude of this charge (E = F/q)? Isn't this field characteristic enough? Of course not. The field strength corresponds to the force of gravity in gravity. But water is attracted by the Earth with the same force both at a meter height and at a hundred meter height. And the electric field, for example, between the plates of a capacitor, a test charge acts with equal strength all the way from the negative plate to the positive one. The charge may be far from the final point of travel, or maybe nearby. It is clear that on a long path a charge (like water in a power plant) is capable of doing more work than on a short path. Therefore, this parameter is necessary - the electric field potential.IN International system units (SI) unit of potential is“Draining” from a “height” of 1 V, a charge of 1 C is capable of doing work of 1 J.
And a load weighing 0.1 kg, falling from a meter height, can do the same work. More precisely, the same amount of work.
And one more thing: we found out the potential of the gravitational field at the level of the second floor window sill. The height difference between the window sill and the ground is 5 meters. It is clear that if there is another one above this window sill, the same 5 meters above the first one, the potential difference between the window sills will be the same 50/sec^2.
In electrostatics, too, “ground” - a conductor with zero potential - is not always taken as zero (base). More often they speak of a “potential difference” between points in an electric field or between conductors.
Let's drop the ball from a certain height. As it falls, it loses potential energy (let me remind you of its formula: Ep = m*g*h, where m is the mass of the ball, g is the acceleration of free fall, h is the height). It is clear that the loss of potential energy occurs due to the parameter h - height, since m and g are constant. However, losing height, the ball gains speed, and therefore kinetic energy Eк = m*v^2/2, where v is speed. And the sum of these two energies - kinetic and potential - at any moment of time is constant (const) and equal to the initial potential energy of the ball: Ep + Ek = const.
Or:m*v^2/2 =m*g*h1 - m*g*h2. Ag*h, as we remember, is the potential of a gravitational field point.
This is also true for a body with charge q and mass m, accelerating in an electric field:
m*v^2/2 = q* φ1- q*φ2
Q* ( φ1-φ2)